In the ennoble power laboratory we examine electrodes and membranes, especially for alkaline electrolysis and fuel cells.
The classic AFC has a narrow gap (instead of a membrane) between two gas diffusion electrodes through which electrolyte liquid is pumped. This ensures very good temperature control of the cell and reaction water can be supplied and removed; the gas spaces always remain dry. The operating principle of an alkaline electrolyzer with an electrolyte gap is shown below:

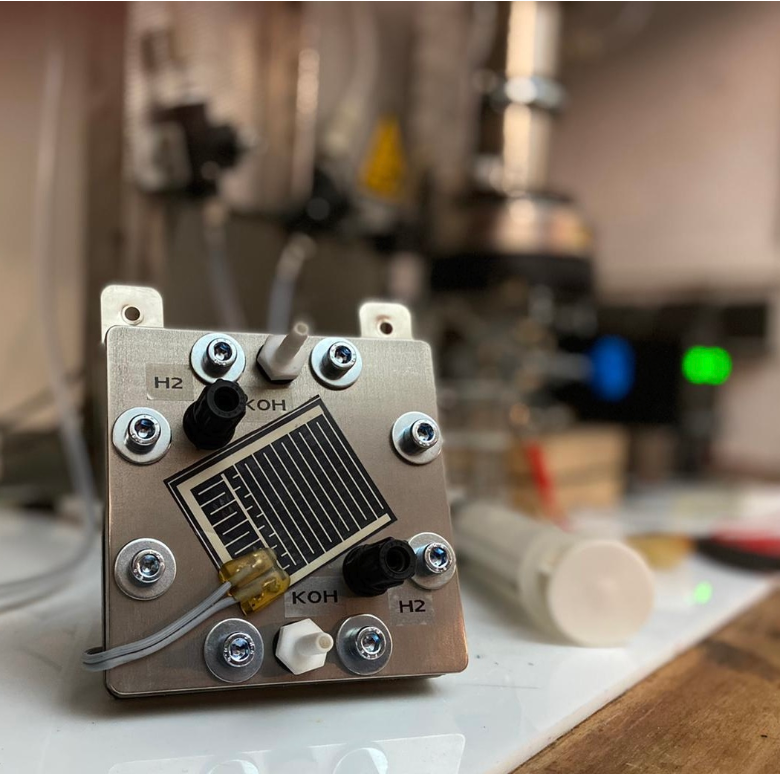
We have developed, tested and protected a reversible alkaline fuel cell. This cell works with an electrolyte gap around 1.5 mm wide, in both electrolysis and fuel cell operation. It can therefore be used as an efficient energy storage device or as an electrolyzer for hydrogen production. Gas diffusion electrodes from Gaskatel from Kassel are used for the structure.
The reversible alkaline fuel cell offers some key advantages:
- Storage efficiencies of up to 60 percent (with PEM fuel cells a maximum of 50 percent)
- Electrolysis efficiency of over 100 percent possible (heat is converted into hydrogen!)
- Switch between modes in seconds – just like a regular battery
- Use of inexpensive catalysts made of nickel – no platinum required
- Inexpensive production from synthetic materials
Do you want to get started with highly efficient alkaline technology, set up a demonstrator, or examine your own electrodes?
Then we will be happy to make you an offer.
Syntropic Number
In a more advanced approach, we look at the connection between energy and information. The syntropy concept states that high-quality forms of energy such as exergy (or syntropy) can not only perform mechanical work but can also “produce” information. This connection is highly relevant – from basic physical research to social systems.
The syntropy index provides information about how efficiently companies process energy and information. This is important for future assessments of sustainability: the more innovative and flexible companies are, the better they will be able to adapt to the rapidly changing circumstances of a future energy landscape. Stodgy structures that are designed to maximize the throughput of fossil fuels will have to take on the great challenge of adapting to new energy sources.

The graphic shows the syntropic number (Syntropie-Index) of some selected companies from different branches of the manufacturing industry. Companies with a high mass throughput of fossil energies (Chemistry / Chemie) therefore have a low syntropy index. Modern companies, on the other hand, which have increasingly switched their production to high-quality electricity from renewable energies, are characterized by a high key figure.
More details are available in a published article:
E. Wagner, Das System Brennstoffzelle, Hanser, 2023
E. Wagner, Ökologisches Wirtschaften 3, 2018 (33)
